WHY ELEMENTS NEED TO BE CLASSIFIED?
The elements need to be classified so that they can be easily studied.
PERIODIC TABLE-
The periodic table arranges all of the known elements in order of increasing atomic number. Order generally coincides with increasing atomic mass. The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies. Elements that lie in the same column on the periodic table (called a "group") have identical valance electron configurations and consequently behave in a similar fashion chemically. For instance, all the group 18 elements are inert, or noble gases.
SOME EARLIER ATTEMPTS TO CLASSIFY ELEMENTS



The elements need to be classified so that they can be easily studied.
PERIODIC TABLE-
The periodic table arranges all of the known elements in order of increasing atomic number. Order generally coincides with increasing atomic mass. The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies. Elements that lie in the same column on the periodic table (called a "group") have identical valance electron configurations and consequently behave in a similar fashion chemically. For instance, all the group 18 elements are inert, or noble gases.
SOME EARLIER ATTEMPTS TO CLASSIFY ELEMENTS
1 METALS AND NON METALS
Among earlier classifications,Lavoisier classified elements into metals and non metals but it was proved to be inadequate.
2 DOBEREINER'S TRIADS
He classified elements into group of 3,such that they had similar properties and the average of atomic masses of first and third elements was equal to the atomic mass of the middle or second element.
Limitations-He could not classify all the known elements into triads.
3 NEWLAND'S LAW OF OCTAVES
He arranged the elements into the increasing order of their atomic masses such that the property of every eighth element was equal to the first element.
Limitations-This law was only applicable on the elements till calcium.
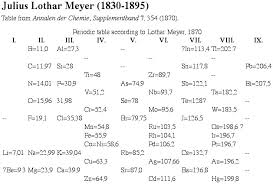
4 LOTHAR MEYER'S ARRANGEMENT
He classified elements according to their atomic volumes.The atomic volume of an element is the volume occupied by 1 mole of an element in its solid state.
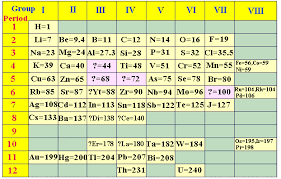
5 MENDELEEV'S PERIODIC TABLE
He classified elements mainly on the basis of their reaction with oxygen and water.
Mendeleev's periodic law
the physical and chemical properties of elements are periodic function of their atomic masses.
Limitations-He could not tell the cause of periodicity.
6 MODERN PERIODIC TABLE-MOSELEY
He classified elements in the increasing order of their atomic numbers.
Modern periodic law
the physical and chemical properties of elements are periodic function of their atomic numbers.
SOURCE-www.livescience.com;modern abc of chemitry[book]
No comments:
Post a Comment