Sunday 29 December 2013
MODERN PERIODIC TABLE VS MENDELEEV's PERIODIC TABLE
MODERN PERIODIC TABLE vs MENDELEEV'S PERIODIC TABLE
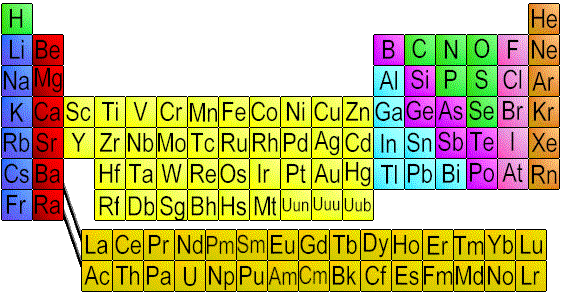

The elements are arranged in grids. The elements are arranged as a list.
The elements are arranged according to their The elements are arranged according to their
increasing atomic number. increasing atomic masses.
Transition metals are placed in different block. Transition metals were placed in groups with non-transition metals
Empty spaces for undiscovered elements were Empty spaces were left for undiscovered
filled. metals
Cause of periodicity is given. Cause of periodic table is not given.
(electronic configuration)
Position of isotopes is logically explained. Position of isotopes is not correct according to Mendeleev's law of atomic masses.
source-modern abc of chemistry(book)
Sunday 22 December 2013
HISTORY OF PERIODIC TABLE
WHY ELEMENTS NEED TO BE CLASSIFIED?
The elements need to be classified so that they can be easily studied.
PERIODIC TABLE-
The periodic table arranges all of the known elements in order of increasing atomic number. Order generally coincides with increasing atomic mass. The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies. Elements that lie in the same column on the periodic table (called a "group") have identical valance electron configurations and consequently behave in a similar fashion chemically. For instance, all the group 18 elements are inert, or noble gases.
SOME EARLIER ATTEMPTS TO CLASSIFY ELEMENTS



The elements need to be classified so that they can be easily studied.
PERIODIC TABLE-
The periodic table arranges all of the known elements in order of increasing atomic number. Order generally coincides with increasing atomic mass. The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies. Elements that lie in the same column on the periodic table (called a "group") have identical valance electron configurations and consequently behave in a similar fashion chemically. For instance, all the group 18 elements are inert, or noble gases.
SOME EARLIER ATTEMPTS TO CLASSIFY ELEMENTS
1 METALS AND NON METALS
Among earlier classifications,Lavoisier classified elements into metals and non metals but it was proved to be inadequate.
2 DOBEREINER'S TRIADS
He classified elements into group of 3,such that they had similar properties and the average of atomic masses of first and third elements was equal to the atomic mass of the middle or second element.
Limitations-He could not classify all the known elements into triads.
3 NEWLAND'S LAW OF OCTAVES
He arranged the elements into the increasing order of their atomic masses such that the property of every eighth element was equal to the first element.
Limitations-This law was only applicable on the elements till calcium.
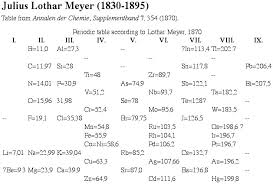
4 LOTHAR MEYER'S ARRANGEMENT
He classified elements according to their atomic volumes.The atomic volume of an element is the volume occupied by 1 mole of an element in its solid state.
5 MENDELEEV'S PERIODIC TABLE
He classified elements mainly on the basis of their reaction with oxygen and water.
Mendeleev's periodic law
the physical and chemical properties of elements are periodic function of their atomic masses.
Limitations-He could not tell the cause of periodicity.
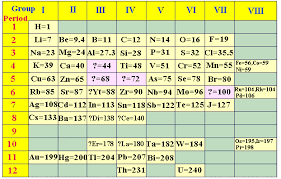
6 MODERN PERIODIC TABLE-MOSELEY
He classified elements in the increasing order of their atomic numbers.
Modern periodic law
the physical and chemical properties of elements are periodic function of their atomic numbers.
SOURCE-www.livescience.com;modern abc of chemitry[book]
Saturday 14 December 2013
Important terms of heredity and evolution
Heredity: The transmission of characters (traits) from the parents to their off springs is called Heredity.
Variation: The differences in the character among the individuals of a species is called variation is necessity for organic evolution.
Both Heredity & variation are fundamental factors in the process of Evolution of an organism.
Genetics: The branch of biology which studies heredity & variation is called genetic.
Terms gene was coined by scientist – Johanssen in 1909.
Chromosomes: Chromosomes are thread like structures present in the nucleus of a cell which contain hereditary information of the cell. They are made up of DNA & proteins.
Gene: Gene is the unit of inheritance. Various genes are located in the chromosomes at fixed position genes are responsible for our characteristic features.
Chemically gene is a segment of a large polynucleotide molecule called de oxyribonucleic acid
DNA: Dexyribo nucleic acid DNA was first isolated by Frederick meisher from the nucleus of the pus cells.It is acidic in nature so the name is nucleic acid. DNA is a macromolecule/polymer which is made up of a large no. of smaller units called Nucleotide. So DNA is a polynucleotide.
Nucleotide is the basic structure unit of DNA.
Sex Determination: The process by which the sex of a per is determined is called sex determination.
The Chromosomes which determine the sex of a person called sex chromosomes which areof 2 types X & Y.
XX combination is always found in females
XY combination is always found in males.
ORGANIC EVOLUTION
Evolution is the sequence of gradual changes which take place in the primitive organisms over millions of yrs. In which non species are provided.
DARWINISM / Theory of Natural Selection (by charles robert darwin)
(a) All the species produce a large no of offsprings but population remains fairly constant due to struggle bt the members of same species & different species for food, space & mate
(b) This struggle eliminates the unfit individuals. (Survival of the fittest)
(c) This gives orgin to variations which pass into progeny & over a long period of time, leads to origin of new species.
Limitations: It could not explain how the variations arise.
Variation: The differences in the character among the individuals of a species is called variation is necessity for organic evolution.
Both Heredity & variation are fundamental factors in the process of Evolution of an organism.
Genetics: The branch of biology which studies heredity & variation is called genetic.
Terms gene was coined by scientist – Johanssen in 1909.
Chromosomes: Chromosomes are thread like structures present in the nucleus of a cell which contain hereditary information of the cell. They are made up of DNA & proteins.
Gene: Gene is the unit of inheritance. Various genes are located in the chromosomes at fixed position genes are responsible for our characteristic features.
Chemically gene is a segment of a large polynucleotide molecule called de oxyribonucleic acid
DNA: Dexyribo nucleic acid DNA was first isolated by Frederick meisher from the nucleus of the pus cells.It is acidic in nature so the name is nucleic acid. DNA is a macromolecule/polymer which is made up of a large no. of smaller units called Nucleotide. So DNA is a polynucleotide.
Nucleotide is the basic structure unit of DNA.
Sex Determination: The process by which the sex of a per is determined is called sex determination.
The Chromosomes which determine the sex of a person called sex chromosomes which areof 2 types X & Y.
XX combination is always found in females
XY combination is always found in males.
ORGANIC EVOLUTION
Evolution is the sequence of gradual changes which take place in the primitive organisms over millions of yrs. In which non species are provided.
DARWINISM / Theory of Natural Selection (by charles robert darwin)
(a) All the species produce a large no of offsprings but population remains fairly constant due to struggle bt the members of same species & different species for food, space & mate
(b) This struggle eliminates the unfit individuals. (Survival of the fittest)
(c) This gives orgin to variations which pass into progeny & over a long period of time, leads to origin of new species.
Limitations: It could not explain how the variations arise.
Saturday 7 December 2013
evolution .....(introduction)
Evolution is the change in the inherited characteristics of biological populations over successive generations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.
All life on Earth is descended from a last universal ancestor that lived approximately 3.8 billion years ago. Repeated speciation and the divergence of life can be inferred from shared sets of biochemical and morphological traits, or by shared DNA sequences. These homologous traits and sequences are more similar among species that share a more recent common ancestor, and can be used to reconstruct evolutionary histories, using both existing species and the fossil record. Existing patterns of biodiversity have been shaped both by speciation and by extinction.
All life on Earth is descended from a last universal ancestor that lived approximately 3.8 billion years ago. Repeated speciation and the divergence of life can be inferred from shared sets of biochemical and morphological traits, or by shared DNA sequences. These homologous traits and sequences are more similar among species that share a more recent common ancestor, and can be used to reconstruct evolutionary histories, using both existing species and the fossil record. Existing patterns of biodiversity have been shaped both by speciation and by extinction.
DISPERSION OF LIGHT
The light rays from the sun consist of seven different colors – red, orange, yellow, green, blue, indigo and violet (ROYGBIV). We see seven different colors when these sun are passed through a glass prism.
The splitting of a ray into its component colors is known as dispersion of light. The band of colors into which the light splits is known as a spectrum. White light consists of photons of various wavelengths (or colors). Photons of different wavelength travel with different speed. The red photon has the longest wavelength and the violet photon has the shortest wavelength. Thus when a white light passes through a prism, different photons cross the medium at different speeds and deviating by different angles. The red color appears at the top of the spectrum because it is bent the least or it is refracted the least.. On the other hand, the violet end of the spectrum is bent the most or refracted most, as it takes longer to traverse the glass medium.
When two prisms are placed with their vertices and bases in the opposite direction, the spectrum of seven colors from the first prism enters the second prism and only a white light comes out.
REASON FOR RECOMBINATION
When white light is passed through a prism,7 bands of colors are coming out of the prism at angle to the direction of incident white ray,the rays are deviated toward the base of the prism
.
If a plane mirroris placed in the path of the band so that the rays are reflected back,all rays retrace their path and emerge out as white ray.
From this it is clear that a white ray passing through a prism is split up into a band and is turned toward the base and If a band of light is incident from the base of the prism they emerge out of the prism as white ray and again turned to ward the base of the prism.
Therefore, when a white ray is passed through the prism they split up into a band and the band, when incident on another prism such that they are incident from the base, they recombine to white ray. In other words, the second prism is inverted so that the band is incident from the base of the second prism.
Subscribe to:
Posts (Atom)